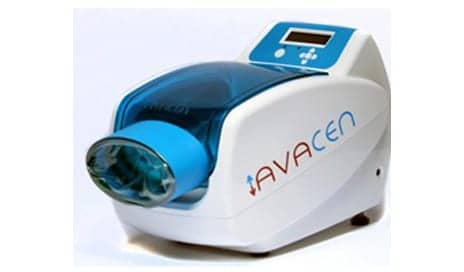
AVACEN Medical, San Diego, announces the receipt of a patent for its AVACEN 100 dry heat therapy medical device and the AVACEN Treatment Method.
According to the company in a media release, the United States patent No 9,192,509 is titled, “Methods and Apparatus for Therapeutic Application of Thermal Energy Including Blood Viscosity Adjustment.”
The AVACEN 100 is an FDA-cleared muscle-relaxation device that is designed to allow patients to treat their entire body from one location—the palm, and works by integrating the health benefits of a dry heat sauna and hyperbaric oxygen treatment, per the release.
With more than 3,000 programmed microprocessor instructions, the device manages the infusion of heat into the circulatory system using negative pressure and a conductive heat source (not LED or Infrared). Arteriovenous anastomosis (AVAs) in the hand act as a portal to infuse heat into the circulatory system, the release explains.
“Overall, our process increases microcirculation. The main functions of microcirculation are to deliver oxygen and nutrients and remove carbon dioxide (CO2) from the deep tissues,” explains AVACEN Medical CEO and inventor, Thomas
“Microcirculation also serves to regulate tissue blood flow. This affects blood pressure and responses to inflammation, including edema (swelling), and chronic pain associated with numerous medical afflictions such as CRPS, Lyme Disease, Fibromyalgia, Rheumatoid Arthritis, Raynaud’s Disease and Carpal Tunnel Syndrome,” he adds.
The AVACEN 100 is FDA-cleared as a Class II heat therapy system for the temporary relief of minor muscle and joint pain and stiffness; the temporary relief of joint pain associated with arthritis, muscle spasm, minor strains and sprains; and muscular relaxation. FDA approval is pending for relief of pain associated with fibromyalgia, per the release.
For more information, visit AVACEN Medical.
[Source(s): AVACEN Medical, PR Newswire]